Exosomal long noncoding RNA MLETA1 promotes tumor progression and metastasis by regulating the miR-186-5p/EGFR and miR-497-5p/IGF1R axes in non-small cell lung cancer
Abstract
Background:
Lung cancer is the most common and deadliest cancer worldwide, and approximately 90% of all lung
cancer deaths are caused by tumor metastasis. Tumor-derived exosomes could potentially promote tumor metastasis
through the delivery of metastasis-related molecules. However, the function and underlying mechanism of exosomal
long noncoding RNA (lncRNA) in lung cancer metastasis remain largely unclear.
Methods:
Cell exosomes were purifed from conditioned media by diferential ultracentrifugation and observed
using transmission electron microscopy, and the size distributions were determined by nanoparticle tracking analysis.
Exosomal lncRNA sequencing (lncRNA-seq) was used to identify long noncoding RNAs. Cell migration and invasion
were determined by wound-healing assays, two-chamber transwell invasion assays and cell mobility tracking. Mice
orthotopically and subcutaneously xenografted with human cancer cells were used to evaluate tumor metastasis
in vivo. Western blot, qRT‒PCR, RNA-seq, and dual-luciferase reporter assays were performed to investigate the poten‑
tial mechanism. The level of exosomal lncRNA in plasma was examined by qRT‒PCR. MS2-tagged RNA afnity purif‑
cation (MS2-TRAP) assays were performed to verify lncRNA-bound miRNAs.
Results:
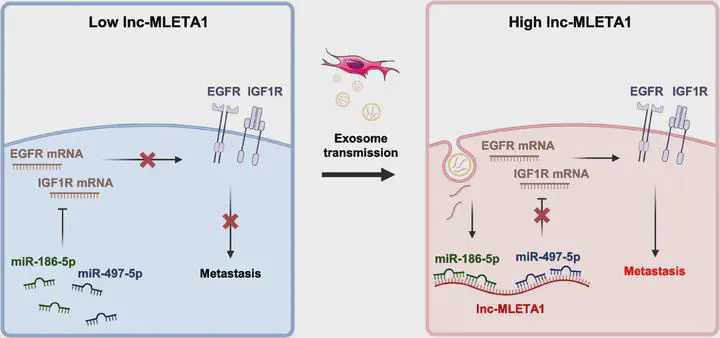
Exosomes derived from highly metastatic lung cancer cells promoted the migration and invasion of lung
cancer cells with low metastatic potential. Using lncRNA-seq, we found that a novel lncRNA, lnc-MLETA1, was upregu‑
lated in highly metastatic cells and their secreted exosomes. Overexpression of lnc-MLETA1 augmented cell migra‑
tion and invasion of lung cancer. Conversely, knockdown of lnc-MLETA1 attenuated the motility and metastasis
of lung cancer cells. Interestingly, exosome-transmitted lnc-MLETA1 promoted cell motility and metastasis of lung
cancer. Reciprocally, targeting lnc-MLETA1 with an LNA suppressed exosome-induced lung cancer cell motility.
Mechanistically, lnc-MLETA1 regulated the expression of EGFR and IGF1R by sponging miR-186-5p and miR-497-5p
to facilitate cell motility. The clinical datasets revealed that lnc-MLETA1 is upregulated in tumor tissues and predicts